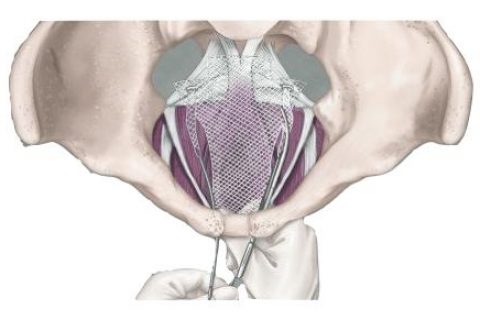
Bladder Sling Complications
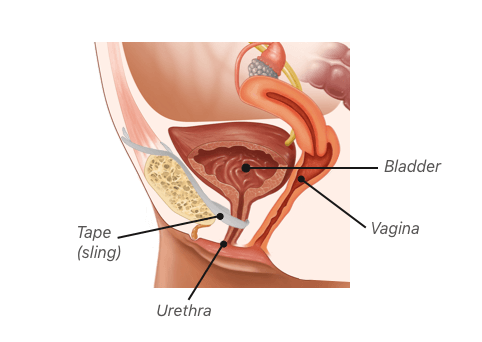
The most common type of bladder sling used to treat stress urinary incontinence is made of plastic mesh. Most women tolerate the procedure well, but mesh slings generally have a higher complication rate than slings made of tissue. Complications may occur during surgery or many years after.
A handful of countries have banned several transvaginal mesh products, including some types of slings, because of complications. Women receive these slings to treat stress urinary incontinence, or SUI. But mesh sling procedures may result in problems, such as bladder perforation, erosion of the mesh into the vagina and painful intercourse. Women who have suffered complications want the devices banned, and some have filed lawsuits against mesh makers.
“I was in terrible pain. My pelvic area was on fire,” Christy Hammond, a woman who received a sling for incontinence, wrote in an article published by Drugwatch. “Sex was out of the question because it hurt so bad. I was getting urinary tract infections (UTIs) on a regular basis.”
“I was in terrible pain. My pelvic area was on fire. Sex was out of the question because it hurt so bad. I was getting urinary tract infections (UTIs) on a regular basis.”
Despite these complications, most doctors prefer mesh slings made of a plastic called polypropylene to treat SUI. The Food and Drug Administration and doctors agree bladder slings are less problematic than mesh for treating pelvic organ prolapse, or POP. In fact, the FDA reclassified surgical mesh for transvaginal repair of pelvic organ prolapse as a high-risk device in January 2016. And in April 2019, the agency stopped the sales of all mesh for POP repair in the United States.
The FDA’s halt on sales does not affect mesh for SUI. Women inured by bladder slings argue that polypropylene has the same risks regardless of where doctors place the product.
Complication rates for bladder slings can vary depending on which study you are reading. This makes it difficult to get an accurate idea of how safe these devices are. Based on the studies it has reviewed, the FDA believes mesh slings for SUI are generally safe and effective.
“Mesh sling surgeries for SUI have been reported to be successful in approximately 70 to 80 percent of women at one year, based on women’s reports and physical exams,” the FDA said on its bladder sling page.
But the agency has found no advantage to using mesh slings.
“Similar effectiveness outcomes are reported following non-mesh SUI surgeries,” the agency said.
Minor and Long-Term Complications
Mesh slings have a higher complication rate than those made of native tissue. For example, synthetic mesh can cause infection, long-term pain and mesh erosion, a complication in which the mesh erodes surrounding tissue. None of these risks are present with slings made of tissue, according to the American College of Obstetrics and Gynecologists.
- Local irritation at wound site
- Infection
- Urinary tract obstruction and urine retention
- Vaginal extrusion
- Erosion through the urethra
- Scarring
- Scar contracture
- Inflammation
- Fistula formation
- Migration of the device
- Pain
- Recurrence of incontinence
- Nerve damage
- Swelling and redness at the wound site
- Vaginal discharge
- Fatigue
- Shortness of breath
- Bleeding
Minor complications, such as bleeding, short-term urinary retention and short-term pain after surgery, are typically easier to resolve. Long-term complications, such as vaginal extrusion, erosion, organ perforation and recurrent infections, can be more difficult to treat.
Women who experience long-term complications may need to undergo revision surgeries, which can be difficult. Infections, such as sepsis, can be life-threatening. Some of these problems can occur because of surgical technique.
A February 2022 study looked at the differences between total removal of mesh and partial removal of mesh. for complications. They found that both methods improved pain, bladder outlet obstruction, mesh erosion or exposure, and lower urinary tract symptoms. However, with complete mesh removal, SUI was more likely to return.
Vaginal Extrusion and Erosion
One of the main concerns with bladder slings has been mesh extrusion or erosion. Extrusion and erosion both refer to mesh forcing its way into the vagina, bladder, urethra or other organ. In these cases, the mesh wears through the tissues.
According to a paper by Dr. Cristiano Mendes Gomes and colleagues, vaginal extrusion rates vary from 0 percent to 1.5 percent for retropubic slings, which are inserted through an incision in the vagina and positioned in a U shape around the urethra. The ends of retropubic slings are maneuvered between the bladder and pubic bone and brought out through incisions above the pubic bone.
For transobturator slings, the vaginal extrusion rates vary from 0 percent to 10.9 percent, according to the paper published in Internal Brazilian Journal of Urology. Known as TOT, this procedure avoids the space between the pubic bone and the bladder. Mesh is inserted through the vagina and the ends are brought out through incisions between the labia and the creases of the thighs.
Additionally, Gomes and colleagues found urethral erosion happened after less than 1 percent of sling surgeries.
Dr. Charles Rardin, a urologist in Providence, Rhode Island, wrote in Ob.Gyn. News that long-term follow-up data indicates erosion occurs after 3 percent to 4 percent of sling placements as opposed to 1 percent as initially believed.
Some studies propose that the risk of erosion may be because of the surgical technique. But mesh that has more contact with the vaginal wall, such as the transobturator sling, may have a higher rate of vaginal erosion.
“Many of the reported cases of erosion occur several years, or longer, after surgery,” Rardin wrote. “It is hard to blame surgical technique for such delayed erosion.”
Women who suffer this complication may have pain during intercourse, incontinence, urgency to urinate, urinary tract infections or obstructions.
Some women may not have any symptoms until the problem becomes more serious. This makes it more important for women who have had bladder sling surgery to follow up with their doctors regularly. Sometimes, erosion occurs just weeks after surgery.
Extrusion Case Study
In case studies published in the journal Urology, Dr. Andrew L. Siegel describes a 48-year-old woman who underwent an ObTape sling procedure. She complained of persistent yellow vaginal discharge, and her husband complained of pain during intercourse.
“Three months postoperatively, she stated that her husband felt ‘teeth’ in her vagina during sexual intercourse,” Siegel wrote.
A pelvic exam revealed mesh extrusion.
In some cases, conservative management of erosion may be possible. For example, some surgeons may prescribe topical estrogen cream to help vaginal tissues heal.
But the woman experienced recurrent incontinence and needed to have the entire sling removed and a new sling placed.
Bladder and Bowel Perforation
Bladder and bowel perforation after mesh placement can result in serious infections and other problems. Perforation happens when mesh or surgical tools injure or cut through an organ.
Bladder perforation is the most commonly reported of these issues. It happens when surgeons puncture the bladder with a needle while placing mesh. But it can also happen when the edges of mesh cut the bladder. John Chang and Dominic Lee with St. George Hospital’s Department of Urology in Australia reported bladder perforation rates of up to 24 percent.
Most of the time, bladder perforation does not cause long-term injury, according to Rardin. Perforation typically occurs because of surgical technique. Surgeons can correct this if they diagnose the injury quickly. In some cases, patients may require a catheter to urinate while the injury heals.
Bowel perforations are far more serious injuries, and fortunately they are rare. These injuries are dangerous because bacteria can leak out of the bowel and cause life-threatening infections. Up until about 2008, the FDA received reports of at least nine bowel perforations. Six of those resulted in death, according to Chang and Lee.
Perforation Case Study
In a 2015 case report published in Case Reports in Obstetrics and Gynecology, authors Peter Kascak and Branislav Kopcan shared the story of a 66-year-old woman whose mesh pierced her small intestine after sling surgery. She had undergone surgery with an experienced specialist for sling placement, and there were no reported complications during surgery.
Initially, she did not suffer fever or other symptom of infection. However, she complained of nausea and vomiting the day after surgery. A CT scan showed inflammation of the abdominal wall, and doctors performed explorative surgery. They discovered the mesh sling had perforated her intestine and the contents of her bowel had leaked into the abdominal cavity. She went into septic shock and died three days after sling placement.
“Although the placement of midurethral sling is a minimally invasive surgery, good diagnostic skills, proper evaluation of indications, safe performance of the procedure, and thorough postsurgical monitoring are paramount for safe and effective outcome of the surgery,” authors wrote.
Kascak and Kopcan reported that intestinal injuries during sling placement were rare, and said that by 2004, the complication had occurred in about 35 out of 700,000 women. Seven of those patients died. Doctors were not aware of the cause until after death in five of those cases.
Complication Rates
The actual rates of mesh sling complications vary widely depending on the study, and several factors may influence reporting rates.
In one study published in Nature in 2017, Kim Keltie and colleagues followed 92,246 women who had had transvaginal mesh slings implanted for incontinence. The study found the complication rate within 30 days or five years of the mesh procedure was about 9.8 percent.
The most common complications after sling procedures are bladder perforation, voiding dysfunction, mesh erosion and post-operative pain, according to Rardin.
“Often times, complications can be significantly more impactful than the original urinary incontinence,” Rardin wrote. “It is important to take the complications of sling placement seriously. Let patients know that their symptoms matter, and that there are ways to manage complications.”
“Often times, complications can be significantly more impactful than the original urinary incontinence. It is important to take the complications of sling placement seriously.”
One of the difficult issues with bladder sling complications is that actual reported complication rates are inconsistent. Low complication rates may give women a false sense of security, while higher rates that occur under less experienced surgeons may scare women.
Dr. Elisabetta Costantini and colleagues published a review in the European Association of Urology journal that found most complications may be underreported. They offered several explanations for the scarcity of major complications in reports: Reports may understate complications; surgeons who have higher complications rates do not answer questionnaires; low-volume and high-volume surgeons experience differences; and surgeons who manage the complications may account for underreporting and over-reporting complication rates.
In addition, major and minor complications are challenging to diagnose and treat even for skilled surgeons, Costantini and colleagues said. These problems can occur during or after surgery. The rate of complications also depends on the technique and sling used in some cases.
Complications During or Shortly After Surgery
Intraoperative or perioperative complications occur during surgery or shortly after. In general, these are rarer. Complication rates range from less than 1 percent to 14 percent, according to Costantini and colleagues. Major complications such as vascular and nerve injuries and gut lesions occurred in less than 1 percent of women. Minor bladder injuries had rates from 0.5 to 14 percent. Significant blood loss occurred in about 2.7 percent to 3.3 percent of women.
Postoperative Complications
The majority of issues that women face with bladder slings occur after surgery. Sometimes, they may occur several years after. Gomes and colleagues reported some of the most recent postoperative complication rates gathered from several studies for retropubic and transobturator slings. Retropubic slings have a higher rate of complications in general, 4.3 percent to 75 percent.
| Complication | Retropubic Sling | Transobturator Sling |
|---|---|---|
| Bleeding | 0.7 to 8 percent | 0 to 2 percent |
| Bladder Injury | 0.7 to 24 percent | 0 to 15 percent |
| Urethral Injury | 0.07 to 0.2 percent | 0.1 to 2.5 percent |
| Urethral Erosion | 0.03 to 0.8 percent | 0.03 to 0.8 percent |
| Intestinal Injury | 0.03 to 0.7 percent | 0 percent |
| Vaginal Erosion | 0 to 1.5 percent | 0 to 10.9 percent |
| Urinary Tract Infection | 7.4 to 13 percent | 7.4 to 13 percent |
| Pain | 4 percent | 9.4 percent |
| Urgency urinating | 0.2 to 25 percent | 0 to 15.6 percent |
| Bladder obstruction | 6 to 18.3 percent | 3 to 11 percent |
| Urinary retention | 4.0 to 19.5 percent | 2.7 to 11 percent |
Complication Rates by Type of Sling
Before undergoing bladder sling surgery, women should ask their doctors about the technique they plan to use. Complication rates may vary depending on the type of mesh sling and technique.
A 2010 study of bladder sling procedures by Z. Chen and colleagues published in Urologia analyzed the outcomes of 187 women who received bladder slings to treat stress urinary incontinence. Authors found that transobturator vaginal tape inside-out (TVT-O) and transobturator vaginal tape out-inside (TOT) are simpler techniques with fewer complications compared to tension-free vaginal tape (TVT).
Women who used TVT had an average hospital stay of five days versus about two days for the TOT group.
The complication rate in the study was:
- 15.6 percent for tension-free vaginal tape (TVT)
- 9.20 percent for transobturator vaginal tape inside-out (TVT-O)
- 8.90 percent for transobturator vaginal tape out-inside (TOT)
Complications from the procedures included discomfort with urinating, bleeding outside blood vessels and dysfunction of lower limbs. TVT was the only procedure associated with bladder perforation. Despite the complication rate, doctors found the slings safe.
“The three tension-free urethral suspension techniques have similar efficacy, all of them are safe and effective procedures for the treatment of female SUI,” authors wrote.
Bladder Sling Complications and Interstitial Cystitis
Some symptoms of bladder sling complications are similar to those of interstitial cystitis (IC), a painful bladder condition that affects millions of Americans. More women than men are likely to get the disease.
Common signs of IC that may overlap with bladder sling complications include: Pelvic and bladder pain, painful sexual intercourse and urinary urgency.
While treating urinary incontinence may involve implanting a mesh bladder sling, doctors typically treat IC with medications instead of surgery. But these medications have their own side effects.
Elmiron (pentosan polysulfatesodium) is the only FDA-approved oral medication to treat the pain and discomfort of IC in the United States. Some recent studies have linked Elmiron to a degenerative vision condition called pigmentary maculopathy.
Pudendal Neuralgia
Persistent pelvic pain that masquerades as IC could be caused by a type of nerve entrapment called pudendal neuralgia. One of the most common causes of pudendal neuralgia is pelvic surgery, such as mesh surgery to repair pelvic organ prolapse or bladder sling surgery.
The incidence goes up if the mesh is problematic and needs to be removed.
“Pudendal nerve compression should always be taken into account when examining and treating patients with symptoms of IC/BPS,” according to Drs. Andreas Gohritz and Arnold Lee Dellon.
Women who have been diagnosed with IC after having bladder sling surgery should speak with their doctor about the potential of pudendal nerve entrapment related to their mesh surgery.
Calling this number connects you with a Drugwatch representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


